Abstract
Introduction: Light chain (AL) amyloidosis is a hematologic neoplasm often diagnosed late after a prolonged course associated with multiple clinical diagnoses (referred to as precursor diagnoses in this abstract) prior to its diagnosis. There is limited information on the epidemiology of AL amyloidosis and its natural history among underrepresented groups. We sought to investigate differences in presentation and co-occurrence of various precursor diagnoses among patients with AL amyloidosis by race and ethnicity.
Methods: We used data from TriNetX, a healthcare research network with de-identified electronic health record data from more than 67 sites across the United States to identify patients with AL amyloidosis. Patients were considered to have AL amyloidosis if they had at least one inpatient visit or two outpatient visits with an associated diagnosis code for AL amyloidosis (ICD-10: E85.81, E85.4, E85.89, E85.9) between 10/01/2015 and 12/31/2020. We further restricted the cohort to those who had at least 2 years of data prior to their AL amyloidosis diagnosis in order to capture information on precursor diagnoses. Individuals with missing race or ethnicity were excluded from this analysis. A total of 30 precursor diagnoses of interest were considered. These were grouped as Clonal (MGUS, smoldering multiple myeloma), Cardiac (e.g., cardiomyopathy, heart failure, dyspnea, arrhythmias), Renal (e.g., proteinuria, chronic kidney disease, nephrotic syndrome), Gastrointestinal (e.g., dysphagia, vomiting, diarrhea, constipation), Neurologic (e.g., peripheral neuropathy, autonomic neuropathy), Multisystemic (e.g., edema, anasarca, syncope, dizziness) and Other (e.g., macroglossia, carpal tunnel syndrome). We compared the prevalence of these between Hispanic (H), Non-Hispanic Black (NHB) and Non-Hispanic White (NHW) patients using Chi-squared or Fisher's exact tests. We also describe the frequency of number of precursor conditions per patient and their co-occurrence for each race and ethnic group. We determined the odds of co-occurrence of the specific precursor diagnoses for the identified population.
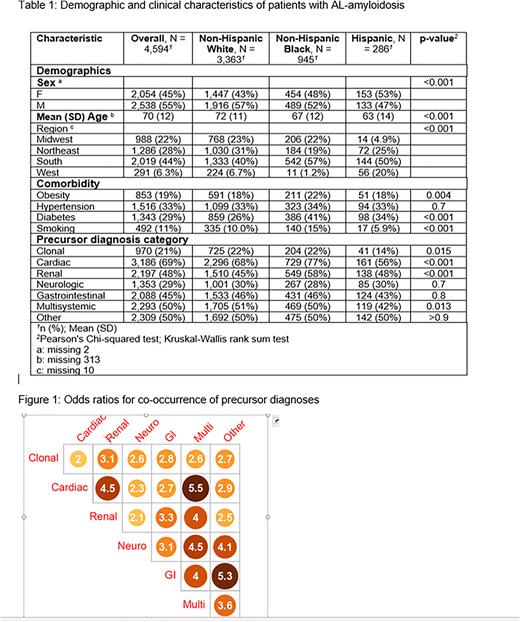
Results: We identified 5,600 AL amyloidosis patients, of whom 4,594 had known race and ethnicity with 3,363 NHW, 945 NHB, and 286 H patients. There were significant differences in age, sex, and geographic region by race and ethnicity. Also, NHB patients had a higher prevalence of comorbidities of obesity, diabetes, and smoking (Table 1). The prevalence of clonal, cardiac, renal, and multisystemic complications was also significantly different by race and ethnicity. The median time between precursor diagnoses and AL amyloidosis diagnosis ranged from 26 - 263 days, depending on the precursor category. On average, Hispanic patients had 4.6 precursors, with 87% of patients having at least one precursor. The NHB patients had an average of 5.9 precursors, with 94% having at least one precursor, and NHW patients had an average of 5.7 precursors, with 99% having at least one precursor. Precursor diagnoses co-occurred in more than one -third of patients for each race and ethnicity group. Figure 1 shows the that having a specific type of precursor diagnoses resulted in 2 times or higher odds of having at least one other type of precursor diagnosis. Analysis adjusted by age, sex, and comorbidities will be shown in the final presentation.
Conclusions: Multiple differences are identified in AL amyloidosis epidemiology and precursor diagnoses by race and ethnicity. Further work is needed to characterize the timing and patterns of co-occurrence of precursor diagnoses in order to develop pathways toward early diagnosis of AL amyloidosis.
Disclosures
D'Souza:Takeda, Sanofi, TeneoBio, Prothena, Caelum Biosciences, Janssen Oncology, Regeneron, Abbvie: Research Funding; Pfizer, Janssen Oncology, Bristol-Myers Squibb/Celgene, Prothena: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal